Learning Outcomes
i. Students will be able to define aldehydes and ketones and identify their functional groups.
ii. Understand the concept of the carbonyl group (C=O) and its role in aldehydes and ketones.
iii. Apply the IUPAC nomenclature system to name aldehydes and ketones systematically.
iv. Explain the structural variations of aldehydes and ketones based on the types of alkyl or aryl groups attached to the carbonyl carbon.
Introduction
Aldehydes and ketones are two important classes of organic compounds characterized by the presence of a carbonyl group (C=O). The carbonyl group is a highly reactive functional group that significantly influences the properties and reactivity of aldehydes and ketones.
i. The Carbonyl Group (C=O)
The carbonyl group is the central feature of aldehydes and ketones. It consists of a carbon atom double-bonded to an oxygen atom. This double bond creates a partial positive charge on the carbon atom and a partial negative charge on the oxygen atom, making the carbonyl group polar and electrophilic.
ii. Aldehydes and Ketones: Functional Group Differences
Aldehydes: Aldehydes have the carbonyl group attached to a carbon atom at the end of a carbon chain. The general formula for aldehydes is R-CHO, where R represents an alkyl or aryl group.
Ketones: Ketones have the carbonyl group attached to a carbon atom within a carbon chain. The general formula for ketones is R-CO-R', where R and R' can be the same or different alkyl or aryl groups.
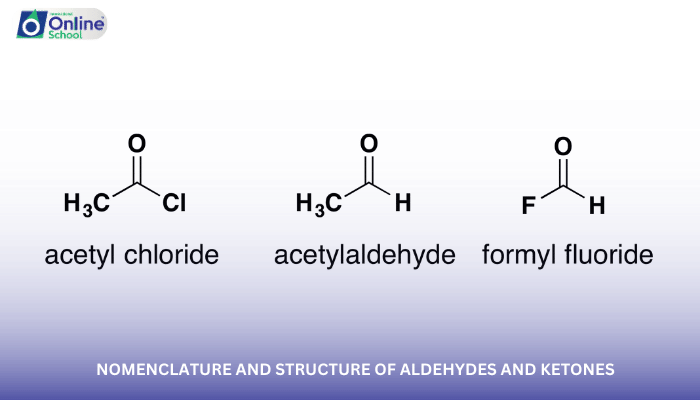
iii. Nomenclature of Aldehydes and Ketones
The IUPAC nomenclature system provides a standardized method for naming aldehydes and ketones.
Aldehyde Nomenclature: The parent chain of an aldehyde is named based on the longest carbon chain containing the carbonyl group. The suffix '-al' is added to the parent chain name. For example, CH3CHO is named methanal, and CH3CH2CHO is named ethanal.
Ketone Nomenclature: The parent chain of a ketone is named based on the longest carbon chain containing the carbonyl group. The prefix 'oxo-' is inserted before the parent chain name, followed by the number of the carbon atom to which the carbonyl group is attached. For example, CH3COCH3 is named 2-propanone, and CH3COCH2CH3 is named 3-pentanone.
iv. Structural Variations of Aldehydes and Ketones
Aldehydes and ketones exhibit structural variations depending on the types of alkyl or aryl groups attached to the carbonyl carbon.
Aliphatic Aldehydes and Ketones: Aldehydes and ketones with alkyl substituents are classified as aliphatic aldehydes and ketones, respectively.
Aromatic Aldehydes and Ketones: Aldehydes and ketones with aryl substituents are classified as aromatic aldehydes and ketones, respectively.
Cyclic Aldehydes and Ketones: Aldehydes and ketones with cyclic structures are classified as cyclic aldehydes and ketones, respectively.
Aldehydes and ketones play significant roles in various biological and industrial processes. Understanding their structure, nomenclature, and functional group properties is essential for comprehending their chemistry and applications.